At initial diagnosis of metastatic disease, central nervous system (CNS) metastases are present in 22–33% of patients with anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC). After crizotinib failure, the incidence can reach up to 70%, compared with 40% in all comers.1 The presence of CNS disease is an indicator of poor prognosis in NSCLC and is associated with a lower quality of life compared with metastasis in other organs. Therefore, optimal treatment, or even prevention of CNS disease, is important. Next-generation ALK inhibitors (ngALKi), including second-generation ALKi (alectinib, ceritinib, brigatinib) and the third-generation ALKi (lorlatinib), have increased potency and higher CNS activity compared to the first-generation ALKi, crizotinib.1 In this editorial, we will discuss the management of patients with ALK+ NSCLC with CNS metastases, with a special focus on the activity of ngALKi.
First-generation versus ngALKi in the prevention of CNS disease
Crizotinib, the first approved ALKi, has superior intracranial disease control rates compared with standard chemotherapy (85% versus 45%, respectively, at 12 weeks). However, the intracranial time to tumour progression (TTP) was not significantly different between the two treatment arms, probably because of the insufficient CNS penetration of crizotinib (cerebrospinal fluid [CSF]/plasma ratio 0.0026).2,3 NgALKi can largely overcome this problem. Interestingly, when used in the first-line setting, alectinib and crizotinib had similar efficacy in controlling
extra-CNS disease. The progression-free survival (PFS) advantage of alectinib could be mainly attributed to its lower rates of CNS progression.1 CNS progression under first-line crizotinib occurred in 45% of cases compared with 12% with alectinib in the ALEX trial (ClinicalTrials.gov identifier, NCT02075840),4 with a cause-specific hazard ratio (HR) for intracranial TTP of 0.18 in favour of alectinib for patients with baseline brain metastases and 0.14 for patients without brain metastases. These results indicate a preventive effect of alectinib on brain metastasis development and progression.5 Similar results were found with brigatinib in the first-line ALTA-1L trial (NCT02737501). CNS progression, as the first site of disease progression, was lower with brigatinib compared with crizotinib (9% versus 19%, respectively, in patients with baseline brain metastases; 1% versus 5%, respectively, in patients without baseline brain metastases).6 These results show that the upfront use of ngALKi, instead of crizotinib, is able to decrease the incidence of brain metastases. In the ASCEND-4 trial (NCT01828099) that evaluated ceritinib in comparison with chemotherapy, CNS progression in patients without baseline brain metastases occurred in 31% of patients, of these patients 30% had an isolated CNS progression.7
How to proceed with treatment in the case of CNS progression on an ALK inhibitor
NgALKi have higher intracranial activity compared with chemotherapy or crizotinib.4–6,8–12 CNS responses occur early, starting from the first evaluation scan,13 and are durable. The duration of response under ngALKi can reach more than 1 year in crizotinib-naïve patients. Importantly, ngALKi are effective also after crizotinib failure, with intracranial overall response rates (ORR) ranging from 27–87% and a median duration of response of several months (Figure 1). After failing at least two lines of ALKi, the third-generation ALKi, lorlatinib, achieved an intracranial ORR of 53% and an intracranial median duration of response of 14.5 months.14 This impressive activity in heavily pre-treated patients is due to the retained potency of lorlatinib to acquired resistant mutations under prior ALKi (including ALK G1202) and because of its better capacity to penetrate into the CNS (CSF drug concentrations of 75% of plasma levels).14,15 The ASCEND-7 study (NCT02336451), which has been specifically designed to assess the CNS response under ceritinib, has also evaluated leptomeningeal disease. This cohort of patients (n=18) was crizotinib pre-treated in 89% of cases and had prior radiotherapy in 61% of cases. The median PFS was 5.2 months and the median overall survival 7.2 months, longer than that established in patients with historical NSCLC (1–3 months).16
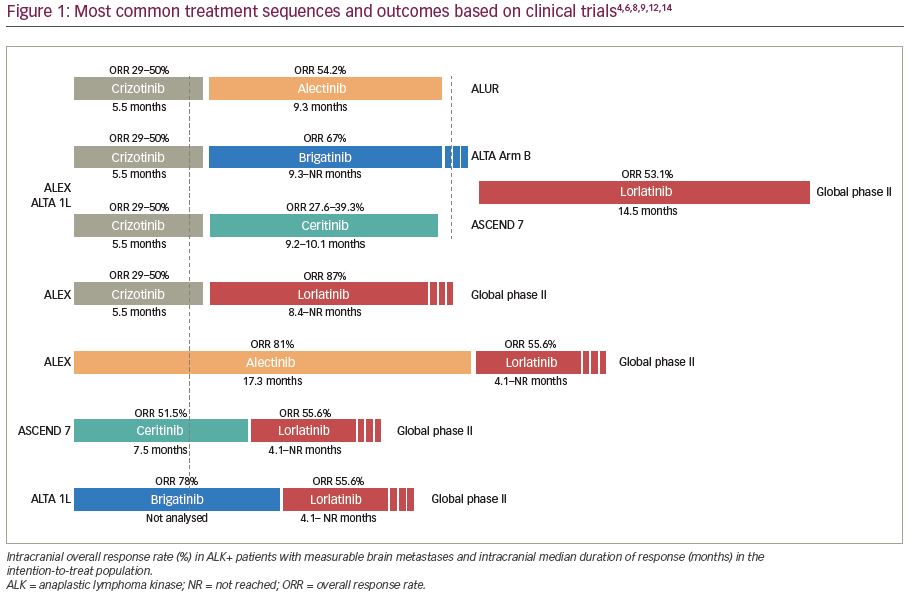
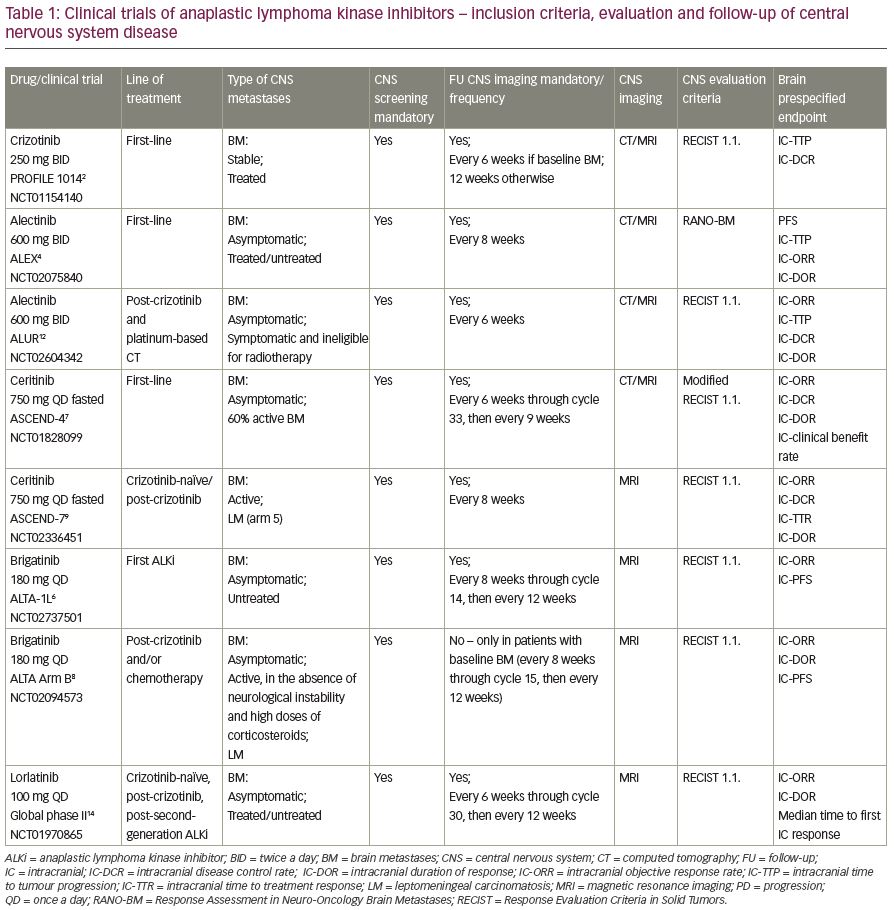
Importantly, data from the above-mentioned clinical trials are not comparable because of different inclusion/evaluation criteria (Table 1).
Patients with CNS relapse might benefit from a molecularly guided treatment sequence
Despite an increase in intracranial activity of ngALKi, CNS disease will often eventually relapse. The sequential use of the third-generation ALKi, lorlatinib, after second-generation ALKi was successful in a global phase II trial (NCT01970865).14 There are currently no clinical trials evaluating a sequential approach between different second-generation ALKi. This might be justified, considering that second-generation ALKi have different ‘sensitivity’ profiles, according to resistance mutations.1 Thus, a molecularly guided approach tailored to secondary mutations could earn additional treatment lines and also increase the chance of obtaining a CNS response. Another situation that could be encountered is the development of isolated CNS progression, a frequent phenomenon with crizotinib, that could also occur with ngALKi.17 For example, in the ASCEND-4 study evaluating first-line ceritinib versus chemotherapy, 48% of patients failing ceritinib developed isolated CNS progression.7 Isolated CNS progression occurs as either a consequence of resistance alterations or insufficient drug penetration. In the latter case, there may be a retention of sensitising alterations.17
The distinction between these two causes of isolated CNS progression would better guide the treatment sequence between alternate second-generation drugs and third-generation drugs or the use of local therapies, such as cranial irradiation. However, performing a molecular profile in patients with isolated CNS progression is challenging, as it should be performed in CSF rather than in plasma. Plasma circulating tumour DNA is negative in ≥50% of cases of isolated CNS progression, depending on the performance of the technique.17 In the absence of resistance alterations, an insufficient drug concentration in the brain might be responsible. However, currently there are only scarce data in several patients that investigate ALKi concentrations in the CSF, and there are no CSF penetration data on ALKi dose escalation.9,15,18 For alectinib dose escalation (900 mg twice daily), intracranial responses have been described in two patients with leptomeningeal disease progression under standard dose of alectinib.19
Switch to another ngALKi might avoid or defer cranial irradiation
In crizotinib-naïve and crizotinib-resistant patients, second- and third-generation ALKi have CNS activity regardless of brain irradiation history. In patients with NSCLC with asymptomatic brain metastases, there were similar intracranial ORRs in irradiated versus non-irradiated patients, as shown by trials of brigatinib (phase I/II trials) or alectinib.5,8 In the ALEX trial, there was a HR for disease progression or death favouring alectinib over crizotinib of 0.34 for previously irradiated patients and 0.44 for patients without prior irradiation.5 Also, patients with active brain metastases had similar intracranial ORR and intracranial median duration of response in irradiated versus non-irradiated tumours in the ASCEND-7 trial, in both crizotinib-naïve or pre-treated patients.9
The availability of effective systemic treatments for sequential use could thus lead to deferring, or even avoiding, whole brain irradiation, which would spare patients the long-term neurocognitive radio-induced side effects. ALK+ patients are usually young, with a long median overall survival of 6.8 years from diagnosis of stage IV disease.20 Similarly, long-term effects of stereotactic radiotherapy, such as radionecrosis, could be seen more frequently. Therefore, anticancer strategies should aim to prolong each line of treatment, without adding significant toxicity. For this purpose, when isolated CNS progression relapse is amenable to local treatment, stereotactic radiotherapy or surgery are usually employed, while continuing the same ALKi. As the incidence of radionecrosis is related to the tumour size, stereotactic radiotherapy should not be delayed too much. It may be interesting to identify patients who will eventually develop early subsequent extra-CNS progression and who might benefit more from a tyrosine kinase inhibitor switch rather than a local treatment. In isolated CNS progression, a positive liquid biopsy might predict subsequent extra-CNS progression, but these data should be validated in prospective studies.17
Due to the availability and performance of ngALKi, patients can have long-term survival despite the presence of brain metastases.20,21 We hope to witness even more rapid improvement in their management as other promising options are being investigated. These include the addition of bevacizumab in the NCT02521051 trial, novel drugs with promising early CNS efficacy data such as entrectinib (NCT03994796, NCT02568267) or ensartinib (NCT03753685), proof of principle studies in patients who will undergo surgery (e.g., NCT02605746), a new lorlatinib trial in patients without measurable extra-CNS lesions (NCT02927340), trials evaluating upfront radiotherapy and ALKi (e.g., NCT04193007), or radiotherapy concurrent with continuation of the ALKi upon isolated CNS progression during ALKi (e.g., the Dutch multicentre platform trial NL6518/NTR6707).
In conclusion, patients with ALK+ NSCLC with brain metastases greatly benefit from ngALKi, irrespective of prior brain irradiation or the presence of brain involvement at baseline, in the first-line, or after failing crizotinib. The sequential ALKi approach is effective in terms of CNS activity and in obtaining long-term survival. Importantly, whole-brain irradiation can often be deferred or avoided, and patients are spared from long-term radio-induced toxicity.